Physical Therapy Equipment Electrotherapy Gel Pads Designed for Muscle Stimulator
See store reviewsEU Medical Device Compliance






Attributes
1 YearWarranty
Online Technical SupportAfter-sale Service
Taiwan, ChinaPlace of Origin
Class IInstrument classification
EverywayBrand Name
SKF50DModel Number
Product name:Electrotherapy gel pads for muscle stimulator
Keywords:Everyway medical 50x50mm round electrode pads
Keywords 2:Rehabilitation therapy pads for stimulator
Keywords 3:physiotherapy adhasive pads for nerve stimulator
Type:Round Adhesive Electrode Pads
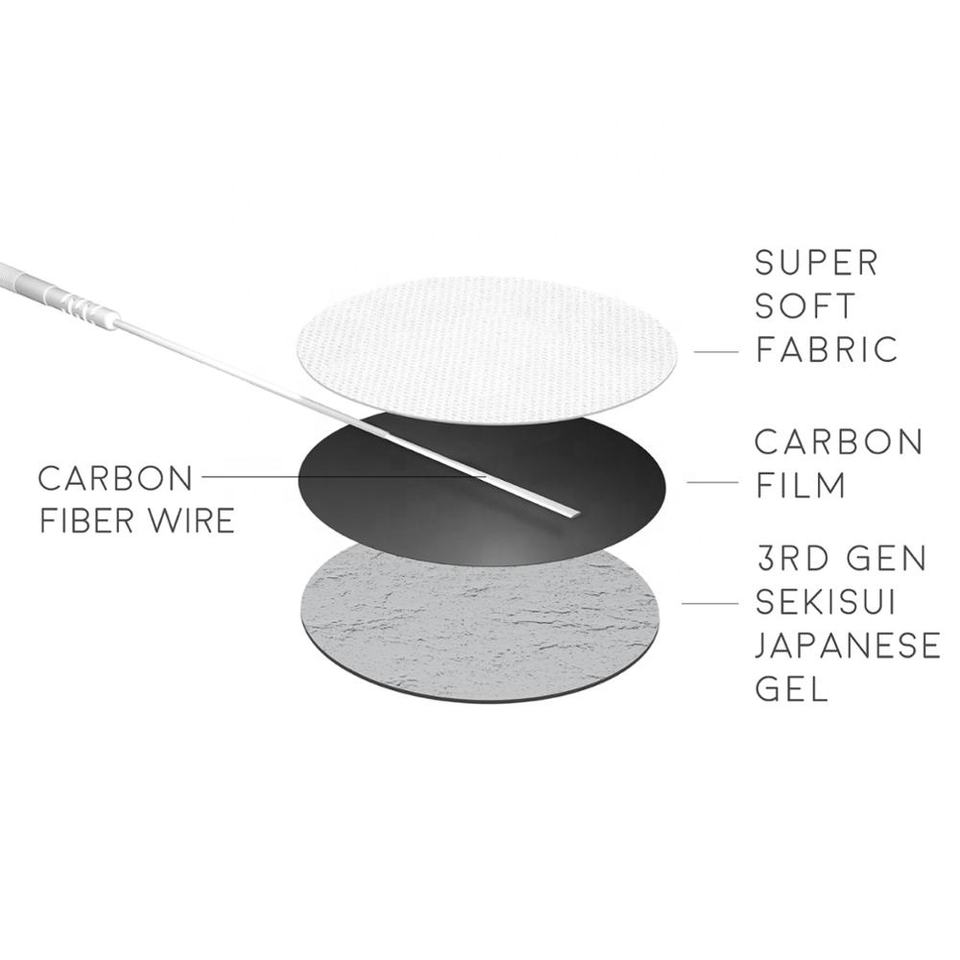
Feature:Carbon Fiber Wire, Carbon Film, Japanese Gel
Material:Carbon Film, Spunlace Cloth
Size:50 x 50 mm
MOQ:400 sets
OEM:Available
Key attributes
Warranty
1 Year
After-sale Service
Online Technical Support
Place of Origin
Taiwan, China
Instrument classification
Class I
Brand Name
Everyway
Model Number
SKF50D
Product name
Electrotherapy gel pads for muscle stimulator
Keywords
Everyway medical 50x50mm round electrode pads
Keywords 2
Rehabilitation therapy pads for stimulator
Keywords 3
physiotherapy adhasive pads for nerve stimulator
Type
Round Adhesive Electrode Pads
Feature
Carbon Fiber Wire, Carbon Film, Japanese Gel
Material
Carbon Film, Spunlace Cloth
Size
50 x 50 mm
MOQ
400 sets
OEM
Available
Packaging and delivery
Selling Units
Multiple of 1,600
Package size per batch
43X31X38 cm
Gross weight per batch
10.500 kg
Lead time
Product descriptions from the supplier
1 batch = 1600 pieces
1600 - 158400 pieces
$0.32
160000 - 238400 pieces
$0.25
240000 - 478400 pieces
$0.22
>= 480000 pieces
$0.17

Sample price: $0.32
Variations
Select nowShipping
4 interest-free payments with
Alibaba.com order protection
Secure payments
Every payment you make on Alibaba.com is secured with strict SSL encryption and PCI DSS data protection protocols
Delivery arranged by supplier
Expect your order to be delivered before scheduled dates or receive a 10% delay compensation
Money-back protection
Claim a refund if your order doesn't ship, is missing, or arrives with product issues

 EU Medical Device Compliance
EU Medical Device Compliance















